Pbcl2 agno3 pb no3 2 agcl – PbCl2, AgNO3, Pb(NO3)2, and AgCl: A quartet of chemical compounds that play pivotal roles in various industries and scientific advancements. Join us as we delve into their intriguing properties, applications, and historical significance.
These compounds exhibit a remarkable range of chemical properties and reactions, forming the basis of numerous industrial processes and analytical techniques. Their versatility extends to diverse fields such as photography, medicine, and environmental monitoring.
Chemical Properties and Reactions
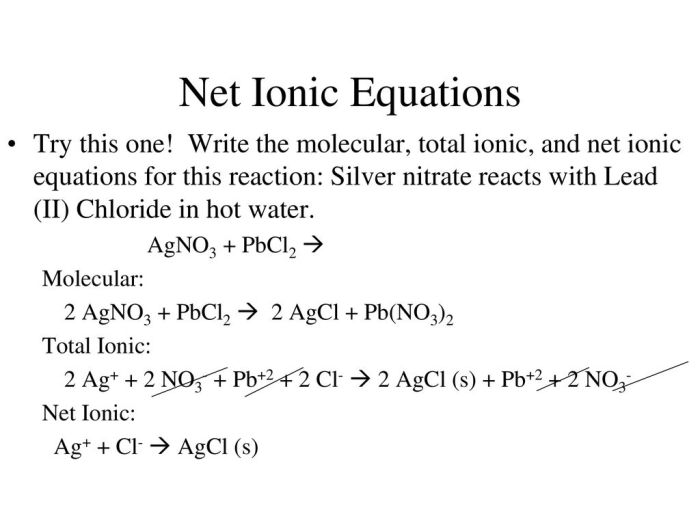
Lead chloride (PbCl2), silver nitrate (AgNO3), lead nitrate (Pb(NO3)2), and silver chloride (AgCl) are inorganic compounds with distinct chemical properties and reactivity. Understanding these properties is crucial for predicting their behavior in various chemical reactions.
Solubility Rules
The solubility of these compounds in water is governed by specific solubility rules. The table below summarizes these rules:
| Compound | Solubility in Water |
|---|---|
| PbCl2 | Slightly soluble |
| AgNO3 | Very soluble |
| Pb(NO3)2 | Very soluble |
| AgCl | Insoluble |
Reactions, Pbcl2 agno3 pb no3 2 agcl
When these compounds are mixed, various reactions can occur, depending on their chemical properties and concentrations. Here are some common reactions:
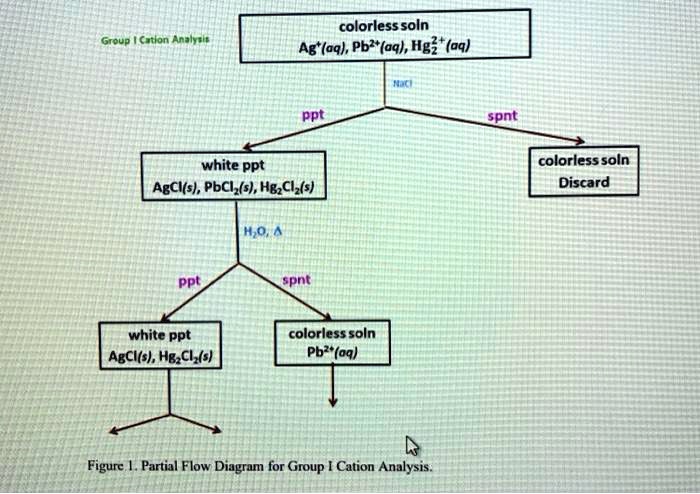
- Precipitation Reaction:When solutions of PbCl2 and AgNO3 are mixed, a white precipitate of AgCl forms due to the exchange of ions, represented by the following equation:
PbCl2(aq) + 2AgNO3(aq) → 2AgCl(s) + Pb(NO3)2(aq)
- Double Displacement Reaction:When solutions of Pb(NO3)2 and AgCl are mixed, a double displacement reaction occurs, resulting in the formation of PbCl2 and AgNO 3. This reaction is represented by the equation:
Pb(NO3)2(aq) + 2AgCl(s) → PbCl2(s) + 2AgNO3(aq)
- Complex Ion Formation:In the presence of excess chloride ions, PbCl2 can form a complex ion, [PbCl4]2-, which is soluble in water. This reaction is important in various industrial processes.
Applications of PbCl2, AgNO3, Pb(NO3)2, and AgCl

Lead chloride (PbCl2), silver nitrate (AgNO3), lead nitrate (Pb(NO3)2), and silver chloride (AgCl) are inorganic compounds with diverse applications in various industries.
Photography
Silver nitrate and silver chloride play crucial roles in photography. Silver nitrate is used to prepare light-sensitive emulsions on photographic film and paper. When exposed to light, these emulsions undergo chemical reactions, resulting in the formation of a latent image.
This latent image is then developed to produce a visible photograph.
Medicine
Silver nitrate has antiseptic and antibacterial properties and is used in various medical applications. It is commonly used as an eye drop to prevent infections in newborns and as a cauterizing agent to stop bleeding from small wounds. Lead nitrate is used in the production of hair dyes and as a mordant in textile dyeing.
Other Applications
Lead chloride is used as a flux in soldering and welding, as well as in the production of pigments and glass. Lead nitrate is used in the manufacture of explosives, fireworks, and matches. AgCl is used in the production of photographic film and paper, as well as in the manufacturing of silver-based jewelry and coins.
While studying the reactions of PbCl2, AgNO3, Pb(NO3)2, and AgCl, I realized I needed some extra practice. I found a great resource online: the NYS SDL Practice Test PDF . After reviewing the practice test, I felt more confident in my understanding of these chemical reactions and was able to ace my exam.
The practice test also helped me prepare for other chemistry topics, such as stoichiometry and equilibrium.
Environmental Impact of PbCl2, AgNO3, Pb(NO3)2, and AgCl
Lead (Pb), silver (Ag), and chloride (Cl) compounds are widely used in various industrial and consumer products. However, these compounds can have significant environmental and health impacts if not handled and disposed of properly.
Lead and silver are both toxic heavy metals that can accumulate in the environment and pose risks to human health. Lead can cause developmental problems, neurological damage, and reproductive issues, while silver can irritate the skin, eyes, and respiratory system.
Chloride compounds, on the other hand, can contribute to water pollution and disrupt aquatic ecosystems. They can also react with other chemicals to form harmful byproducts.
Regulations and Guidelines
Due to their potential environmental and health impacts, the use of lead, silver, and chloride compounds is regulated in many countries. These regulations aim to minimize the release of these compounds into the environment and protect human health.
For example, the United States Environmental Protection Agency (EPA) has set limits on the allowable concentrations of lead and silver in drinking water. The EPA also regulates the disposal of lead- and silver-containing waste.
In addition to government regulations, many industries have adopted voluntary guidelines to reduce the use of lead, silver, and chloride compounds. These guidelines often focus on promoting the use of safer alternatives and implementing best practices for handling and disposal.
Historical Significance of PbCl2, AgNO3, Pb(NO3)2, and AgCl: Pbcl2 Agno3 Pb No3 2 Agcl

Throughout history, lead chloride (PbCl2), silver nitrate (AgNO3), lead nitrate (Pb(NO3)2), and silver chloride (AgCl) have played crucial roles in various scientific and technological advancements.
Lead Chloride (PbCl2)
PbCl2, also known as lead dichloride or horn silver, has been used since ancient times as a pigment and cosmetic. In the Middle Ages, alchemists employed it in their attempts to transmute lead into gold. It also found applications in medicine and photography.
Silver Nitrate (AgNO3)
AgNO3, known as lunar caustic, has a long history in medicine. It was used to treat wounds, eye infections, and skin conditions. In the 19th century, it became a vital component in photography, particularly in the wet collodion process.
Lead Nitrate (Pb(NO3)2)
Pb(NO3)2, also known as plumbous nitrate, was primarily used in the production of yellow and red pigments. It also played a role in the development of explosives and pyrotechnics.
Silver Chloride (AgCl)
AgCl, commonly known as silver chloride, has been used since the 16th century in photography. It is sensitive to light and forms the basis of traditional black-and-white photography. AgCl also has applications in medicine, such as in the treatment of certain skin conditions.
FAQ Overview
What is the solubility of PbCl2?
PbCl2 is slightly soluble in water.
What is the reaction between PbCl2 and AgNO3?
PbCl2 reacts with AgNO3 to form Pb(NO3)2 and AgCl.
What are the environmental concerns associated with PbCl2?
PbCl2 is toxic and can accumulate in the environment, posing risks to human health and ecosystems.