Embark on an enlightening journey into the realm of chemistry with our comprehensive moles to mass conversion worksheet. This invaluable resource empowers students to delve into the fundamental concepts of moles and mass, unlocking the secrets of their intricate relationship.
Through a series of engaging examples and step-by-step guidance, this worksheet fosters a deep understanding of Avogadro’s number and its pivotal role in converting between the two units. Prepare to unravel the mysteries of chemical stoichiometry and witness the seamless transformation of moles into mass.
1. Moles to Mass Conversion Basics: Moles To Mass Conversion Worksheet
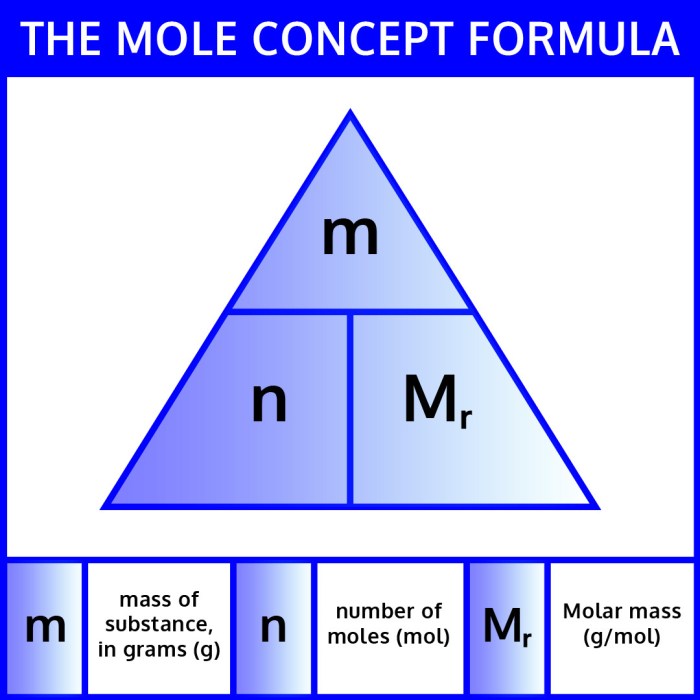
In chemistry, the mole is a fundamental unit of measurement that represents a specific amount of a substance. It is defined as the amount of substance that contains exactly 6.02214076 x 10 23elementary entities (atoms, molecules, ions, or electrons). The mass of a substance is the total amount of matter it contains, typically expressed in grams (g).
The relationship between moles and mass is given by the molar mass, which is the mass of one mole of a substance. Molar mass is expressed in grams per mole (g/mol). To convert between moles and mass, you can use the following formula:
Mass (g) = Moles (mol) x Molar Mass (g/mol)
Examples of Moles to Mass Conversion, Moles to mass conversion worksheet
Example 1:Convert 2.5 moles of sodium chloride (NaCl) to grams.
Step 1:Find the molar mass of NaCl: 22.99 g/mol (Na) + 35.45 g/mol (Cl) = 58.44 g/mol
Step 2:Use the formula: Mass (g) = 2.5 mol x 58.44 g/mol = 146.1 g
Example 2:Convert 50.0 g of glucose (C 6H 12O 6) to moles.
Step 1:Find the molar mass of glucose: 6(12.01 g/mol) + 12(1.01 g/mol) + 6(16.00 g/mol) = 180.16 g/mol
Step 2:Use the formula: Moles (mol) = 50.0 g / 180.16 g/mol = 0.277 mol
General Inquiries
What is the definition of a mole?
A mole is the SI unit of amount of substance, defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
How do I convert moles to mass?
To convert moles to mass, multiply the number of moles by the molar mass of the substance.
What is Avogadro’s number?
Avogadro’s number is the number of elementary entities (atoms, molecules, ions, or electrons) in one mole of a substance. It is approximately 6.022 × 10^23.