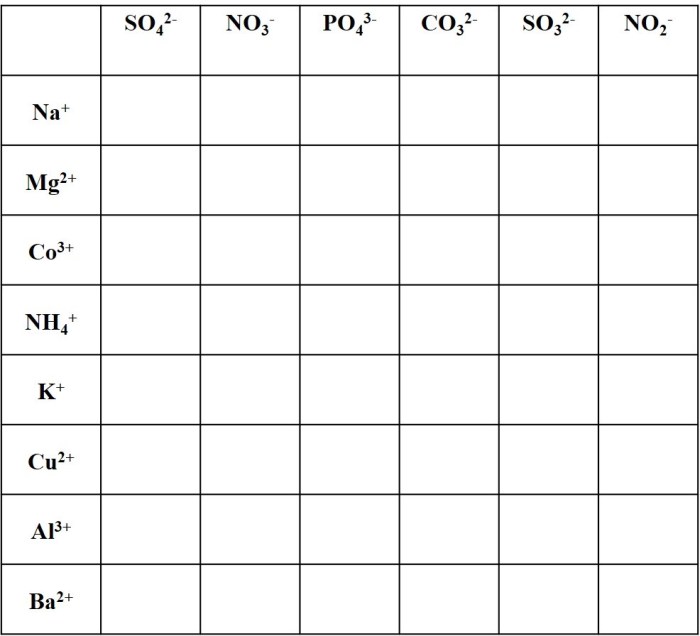
Polyatomic ions worksheet with answers – Embark on a captivating journey into the realm of polyatomic ions with our comprehensive worksheet and answer key. This resource unveils the intricacies of these fascinating chemical entities, empowering you to navigate their complexities with confidence.
Delve into the fundamental concepts, naming conventions, and unique properties of polyatomic ions. Discover their influence on compound behavior and master the art of writing balanced chemical formulas that incorporate these ions.
Polyatomic Ions: Basic Concepts

Polyatomic ions are ions composed of multiple atoms that are covalently bonded together. They carry a net electric charge and behave as a single unit in chemical reactions.
Polyatomic ions are typically formed when a molecule gains or loses electrons. The number of electrons gained or lost determines the charge of the ion. For example, the sulfate ion (SO 42-) is formed when a sulfur atom gains two electrons.
Naming Polyatomic Ions, Polyatomic ions worksheet with answers
Polyatomic ions are named according to the following rules:
- The name of the ion ends in “-ate” if the ion contains the most oxygen atoms possible.
- The name of the ion ends in “-ite” if the ion contains one less oxygen atom than the “-ate” ion.
- The prefix “hypo-” is used to indicate that the ion contains one less oxygen atom than the “-ite” ion.
- The prefix “per-” is used to indicate that the ion contains one more oxygen atom than the “-ate” ion.
For example, the sulfate ion (SO 42-) is named because it contains the most oxygen atoms possible for a sulfur-containing ion. The nitrite ion (NO 2–) is named because it contains one less oxygen atom than the nitrate ion (NO 3–).
Properties of Polyatomic Ions
Polyatomic ions have a number of unique properties that influence their behavior in compounds.
- Polyatomic ions are typically larger than monatomic ions.
- Polyatomic ions are typically more soluble in water than monatomic ions.
- Polyatomic ions can form complex ions with metal ions.
These properties make polyatomic ions important in a wide variety of chemical reactions.
Writing Formulas with Polyatomic Ions
When writing chemical formulas with polyatomic ions, it is important to remember that the charge of the ion must be balanced by the charges of the other ions in the compound.
For example, to write the formula for sodium sulfate, we must first determine the charge of the sulfate ion. The sulfate ion has a charge of 2-, so the formula for sodium sulfate is Na 2SO 4.
Identifying Polyatomic Ions in Chemical Equations
Polyatomic ions can be identified in chemical equations by their characteristic names and charges.
For example, in the following equation, the sulfate ion is identified by its name (sulfate) and its charge (2-):
2 Na + SO 42-→ Na 2SO 4
Polyatomic Ion Concentration Calculations
The concentration of polyatomic ions in solutions can be calculated using the same methods that are used to calculate the concentration of monatomic ions.
For example, the concentration of the sulfate ion in a solution can be calculated using the following equation:
[SO 42-] = M / V
where M is the molarity of the solution and V is the volume of the solution in liters.
Applications of Polyatomic Ions
Polyatomic ions are used in a wide variety of applications, including:
- Fertilizers
- Detergents
- Batteries
- Paints
Polyatomic ions are essential for many of the chemical reactions that occur in everyday life.
Commonly Asked Questions: Polyatomic Ions Worksheet With Answers
What is the definition of a polyatomic ion?
A polyatomic ion is a group of atoms that carries a net electrical charge and behaves as a single unit.
How do I name polyatomic ions?
Polyatomic ions are named using specific rules that consider their elemental composition and charge.
What are the physical and chemical properties of polyatomic ions?
Polyatomic ions exhibit unique physical and chemical properties that influence their behavior in compounds and solutions.