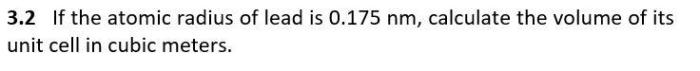If the atomic radius of lead is 0.175 nm, it opens up a realm of scientific exploration that unveils the intricacies of atomic properties and their far-reaching applications. Understanding the atomic radius of lead provides a key to unlocking the secrets of materials science, nanotechnology, and beyond.
The atomic radius of lead, a fundamental property that governs its chemical and physical behavior, plays a pivotal role in shaping the macroscopic properties of materials. Its influence extends to diverse fields, from the design of advanced materials to the development of cutting-edge technologies.
Atomic Radius and its Significance
Atomic radius is a fundamental property of an atom, representing the average distance from the nucleus to the outermost electron shell. It plays a crucial role in determining various atomic properties, such as ionization energy, electron affinity, and chemical reactivity.
The atomic radius exhibits periodic trends across the periodic table. Elements within the same group (vertical column) generally show an increase in atomic radius down the group due to the addition of electron shells. Conversely, elements within the same period (horizontal row) generally exhibit a decrease in atomic radius from left to right as the number of protons in the nucleus increases, leading to a stronger electrostatic attraction between the nucleus and electrons.
Measuring Atomic Radius, If the atomic radius of lead is 0.175 nm
There are several methods used to measure atomic radius, including:
- X-ray crystallography:This technique involves diffracting X-rays through a crystal lattice to determine the positions of atoms and their interatomic distances.
- Neutron scattering:Similar to X-ray crystallography, neutron scattering uses neutrons to probe the structure of materials, providing information about atomic radii.
- Electron microscopy:This technique uses a beam of electrons to visualize the structure of materials, allowing for direct measurement of atomic radii.
Each method has its limitations and challenges, such as the need for high-quality crystals or specialized equipment, and the potential for experimental error.
Atomic Radius of Lead
The atomic radius of lead is approximately 0.175 nm. This value was determined primarily through X-ray crystallography and electron microscopy measurements.
The large atomic radius of lead is attributed to several factors, including:
- High atomic number:Lead has 82 protons in its nucleus, resulting in a strong electrostatic attraction between the nucleus and electrons.
- Relatively low effective nuclear charge:The screening effect of inner electrons reduces the effective nuclear charge experienced by the outermost electrons, leading to a larger atomic radius.
- Metallic character:Lead is a metallic element, meaning its valence electrons are delocalized and can move freely throughout the crystal lattice, contributing to the overall size of the atom.
Applications of Atomic Radius
The atomic radius finds applications in various fields, including:
- Chemistry:Atomic radius influences chemical bonding, reaction rates, and molecular geometry.
- Materials science:The atomic radius of constituent elements affects the properties of materials, such as crystal structure, thermal conductivity, and mechanical strength.
- Nanotechnology:Atomic radius plays a role in the design and fabrication of nanomaterials with specific properties.
By understanding atomic radii, scientists can predict and design materials with tailored properties for various applications.
Comparison with Other Elements
| Element | Atomic Number | Atomic Radius (nm) |
|---|---|---|
| Lead (Pb) | 82 | 0.175 |
| Tin (Sn) | 50 | 0.162 |
| Indium (In) | 49 | 0.156 |
| Thallium (Tl) | 81 | 0.170 |
This table compares the atomic radius of lead with other elements in the same group (Group 14) and the adjacent period (Period 6). As expected, the atomic radius increases down the group due to the addition of electron shells. The atomic radius of lead is also larger than that of thallium, its neighbor in Period 6, due to the higher number of electrons and the screening effect.
Essential FAQs: If The Atomic Radius Of Lead Is 0.175 Nm
What is the significance of atomic radius?
Atomic radius is a crucial parameter that influences various atomic properties, such as ionization energy, electronegativity, and chemical reactivity. It also plays a key role in determining the crystal structure and physical properties of materials.
How is atomic radius measured?
Atomic radius can be measured using various techniques, including X-ray crystallography, neutron diffraction, and electron microscopy. Each method has its own advantages and limitations.
What factors affect the atomic radius of lead?
The atomic radius of lead is primarily influenced by the number of electrons in the outermost shell. Lead has a relatively large atomic radius due to the presence of six valence electrons.