Unit chemical bonding covalent bonding – ws #3 – Embarking on a journey into the realm of chemical bonding, we delve into the intricacies of covalent bonding, a fundamental concept that governs the formation of molecules and shapes the properties of matter. This exploration, designated as Unit Chemical Bonding: Covalent Bonding – WS #3, will unravel the nature of covalent bonds, their types, properties, and diverse applications.
Covalent bonding arises from the sharing of electron pairs between atoms, giving rise to a myriad of molecular structures. This sharing phenomenon manifests in various bond types, including single, double, and triple bonds, each exhibiting distinct characteristics and influencing the overall properties of the molecule.
Covalent Bonding: Definition and Nature: Unit Chemical Bonding Covalent Bonding – Ws #3
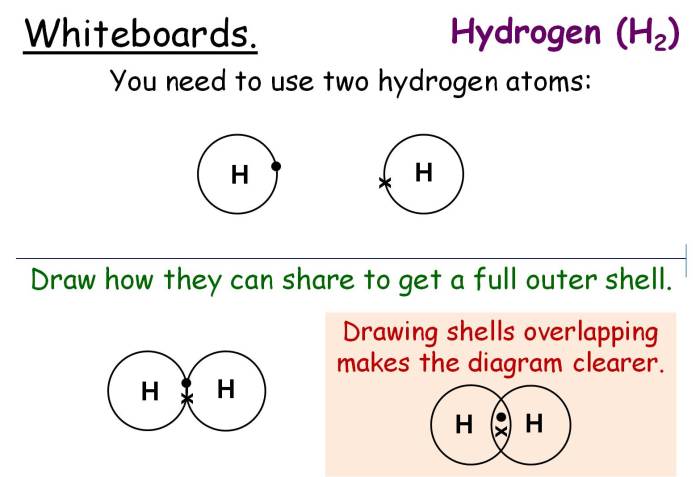
Covalent bonding is a type of chemical bond that involves the sharing of electron pairs between atoms. It is a fundamental concept in chemistry, explaining the formation and properties of many molecules and compounds.
In a covalent bond, the atoms involved share one or more pairs of electrons, creating a strong attraction between them. This sharing results in a stable molecular structure and determines the properties of the compound.
Types of Covalent Bonds, Unit chemical bonding covalent bonding – ws #3
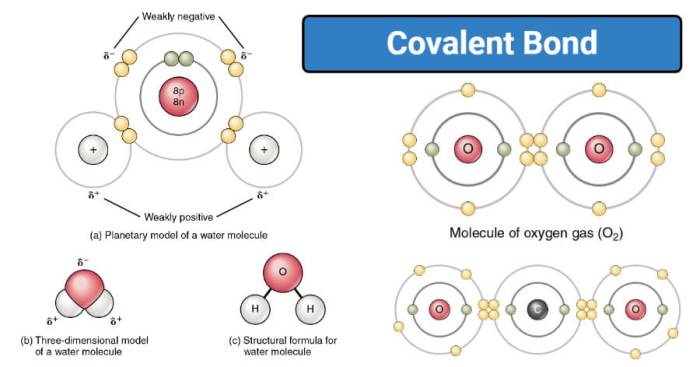
- Nonpolar Covalent Bonds:Formed between atoms of the same element, where the electrons are shared equally. The electronegativity difference between the atoms is negligible.
- Polar Covalent Bonds:Formed between atoms of different elements, where the electrons are not shared equally. The electronegativity difference between the atoms creates a partial positive charge on one atom and a partial negative charge on the other.
Q&A
What is the key characteristic of a covalent bond?
Covalent bonds are formed by the sharing of electron pairs between atoms.
How does electronegativity influence covalent bond polarity?
Electronegativity determines the unequal distribution of electrons in a covalent bond, resulting in bond polarity.
Provide an example of a nonpolar covalent bond.
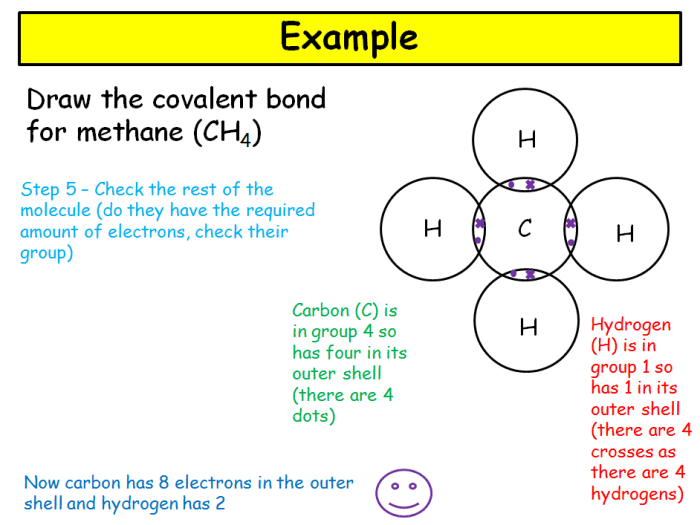
The covalent bond in methane (CH4) is nonpolar due to the equal sharing of electrons between the carbon and hydrogen atoms.