Rank the following compounds in order of increasing boiling point. This task delves into the captivating realm of intermolecular forces and molecular structure, revealing their profound influence on the behavior of substances at their boiling points.
As we embark on this journey, we will uncover the factors that govern the strength of intermolecular interactions, exploring how they shape the physical properties of compounds and ultimately determine their boiling points.
Boiling Point Overview
The boiling point of a substance is the temperature at which its vapor pressure equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid is a characteristic property that depends on the strength of the intermolecular forces between the molecules of the liquid.
Factors that influence boiling point include:
- Molecular weight: Heavier molecules have stronger intermolecular forces and higher boiling points.
- Polarity: Polar molecules have stronger intermolecular forces than nonpolar molecules and higher boiling points.
- Hydrogen bonding: Hydrogen bonding is a particularly strong type of intermolecular force that can significantly increase the boiling point of a liquid.
Intermolecular Forces
Intermolecular forces are the forces that act between molecules. The strength of these forces determines the physical properties of a substance, such as its boiling point.
There are three main types of intermolecular forces:
- Dipole-dipole forces: These forces occur between polar molecules that have a permanent dipole moment.
- London dispersion forces: These forces occur between all molecules, regardless of their polarity. They are caused by the temporary fluctuations in the electron distribution of a molecule.
- Hydrogen bonding: This is a particularly strong type of dipole-dipole force that occurs between molecules that have a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
The strength of intermolecular forces increases in the order: London dispersion forces< dipole-dipole forces < hydrogen bonding.
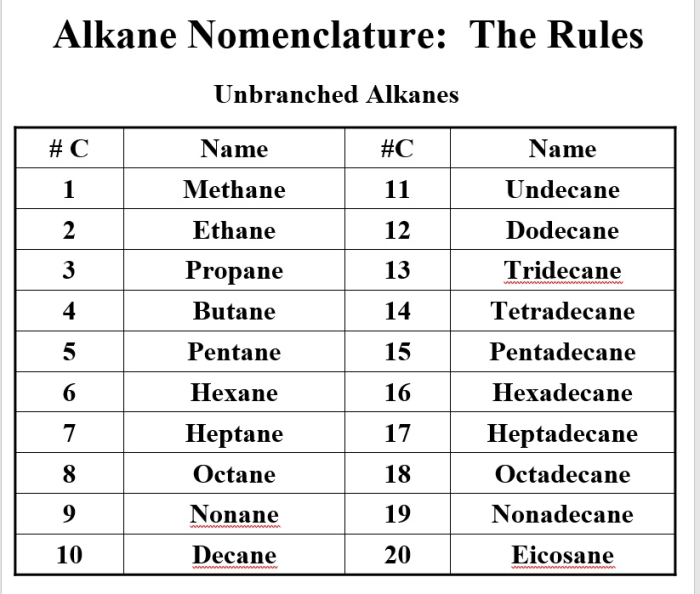
Molecular Structure and Shape: Rank The Following Compounds In Order Of Increasing Boiling Point.
The molecular structure and shape of a compound can also affect its boiling point. Compounds with more compact structures and fewer branches have stronger intermolecular forces and higher boiling points.
For example, n-butane has a higher boiling point than isobutane because n-butane has a more compact structure.
Example Compounds
The following table lists some common compounds and their boiling points:
| Compound | Formula | Boiling Point (°C) |
|---|---|---|
| Methane | CH4 | -161.6 |
| Ethane | C2H6 | -88.6 |
| Propane | C3H8 | -42.1 |
| n-Butane | C4H10 | -0.5 |
| Isobutane | C4H10 | -11.7 |
| Water | H2O | 100.0 |
| Ethanol | C2H5OH | 78.3 |
| Benzene | C6H6 | 80.1 |
As you can see from the table, the boiling points of the compounds increase with increasing molecular weight and polarity.
Applications
Understanding boiling points is important for a variety of applications, including:
- Chemical engineering: Boiling points are used to design and operate chemical processes.
- Petroleum refining: Boiling points are used to separate different components of petroleum.
- Food processing: Boiling points are used to control the cooking process.
- Pharmaceutical industry: Boiling points are used to develop and manufacture drugs.
Quick FAQs
What is the significance of boiling point?
Boiling point is a crucial physical property that provides insights into the intermolecular forces within a substance. It serves as a benchmark for various chemical processes and is essential for understanding the behavior of compounds in different applications.
How do intermolecular forces affect boiling point?
Intermolecular forces are the attractive forces between molecules. The strength of these forces directly influences the boiling point of a substance. Stronger intermolecular forces require more energy to overcome, resulting in higher boiling points.